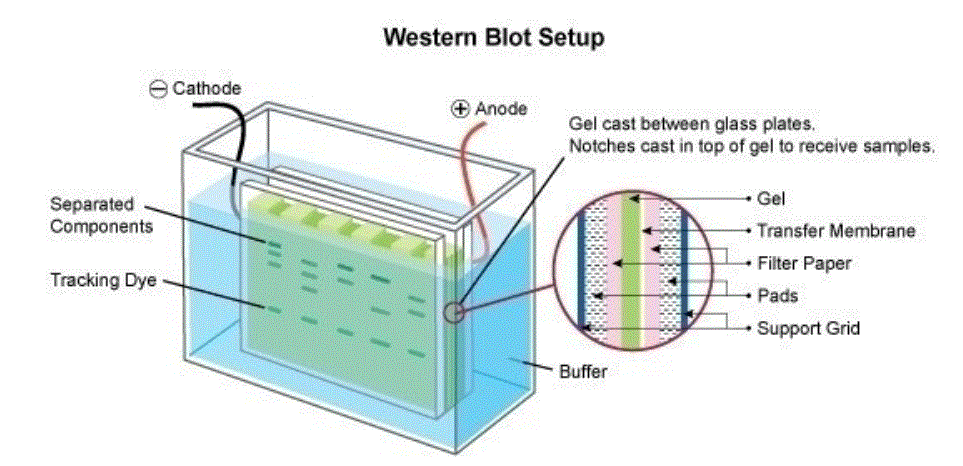
Western Blotting (also referred to as immunoblotting) is a technique used for analysis of individual proteins within a protein mix
(e.g. a cell lysate). In Western blotting (immunoblotting) the protein mix is applied to a gel electrophoresis at a carrier matrix (SDS-PAGE, native PAGE, isoelectric focusing, 2D gel electrophoresis, etc.) to sort the proteins by dimension , charge, or other differences in individual protein bands. The separated protein bands are then transferred to a carrier membrane (e.g.
nitrocellulose, nylon or PVDF). This process is known as blotting. As they’ve been separated due to interactions with fees, the proteins stick to the membrane in the exact same routine. The proteins within this immunoblot are then available for antibody binding for detection.
The antibodies are conjugated with fluorescent or radioactive tags or enzymes that give a subsequent response with an applied reagent, leading to a coloring or emission of light, enabling detection.
The term Western Blotting is based on a play of words. The southern blot, that can be a method to detect DNA sequences, is named after Ed Southern, who first described this process.
The western blot (immunoblot), in addition to the northern blot (for RNA detection), play the significance of this name.
